Recently, researchers from the School of Chemistry and Chemical Engineering have made a significant breakthrough at the intersection of interfacial chemistry and green catalysis. Their findings, titled “Electrode Fouling by Gas Bubbles Enables Catalyst-Free Hydrogen Peroxide Synthesis”, have been published in the internationally renowned journal Journal of the American Chemical Society (JACS). Jiangsu University is listed as the primary affiliation. The study was led by doctoral candidate Xiaoxue Song, with Professors Long Zhang (Jiangsu University), Xinxing Zhang (Nankai University), and Simone Ciampi (Curtin University, Australia) serving as co-corresponding authors.
This work uncovers a novel mechanism whereby gas bubbles immobilized on porous carbon electrodes create gas–liquid–solid triple-phase boundaries that enrich hydroxide ions (OH−) and lower their oxidation energy barrier. Simultaneously, the hydrophobic interface of the bubbles suppresses overoxidation and promotes electron transfer to oxygen molecules (O2), collectively enhancing H2O2 production efficiency. Operating at +1.1 V (vs. Ag/AgCl), the system achieves a H2O2 yield of 8.98 mM in one hour without the need for noble metals or other functional catalysts.
Through a combination of electron paramagnetic resonance (EPR), isotope labeling, in situ infrared spectroscopy, and density functional theory (DFT) calculations, the researchers identified and validated three synergistic pathways for H2O2 formation: electrochemical oxidation of OH−, electrochemical oxidation of H2O, and chemical reduction of O2. The study not only challenges the conventional belief that surface bubbles hinder electrochemical reactions, but also provides a new conceptual framework and platform for sustainable synthesis, clean energy conversion, and environmental remediation.
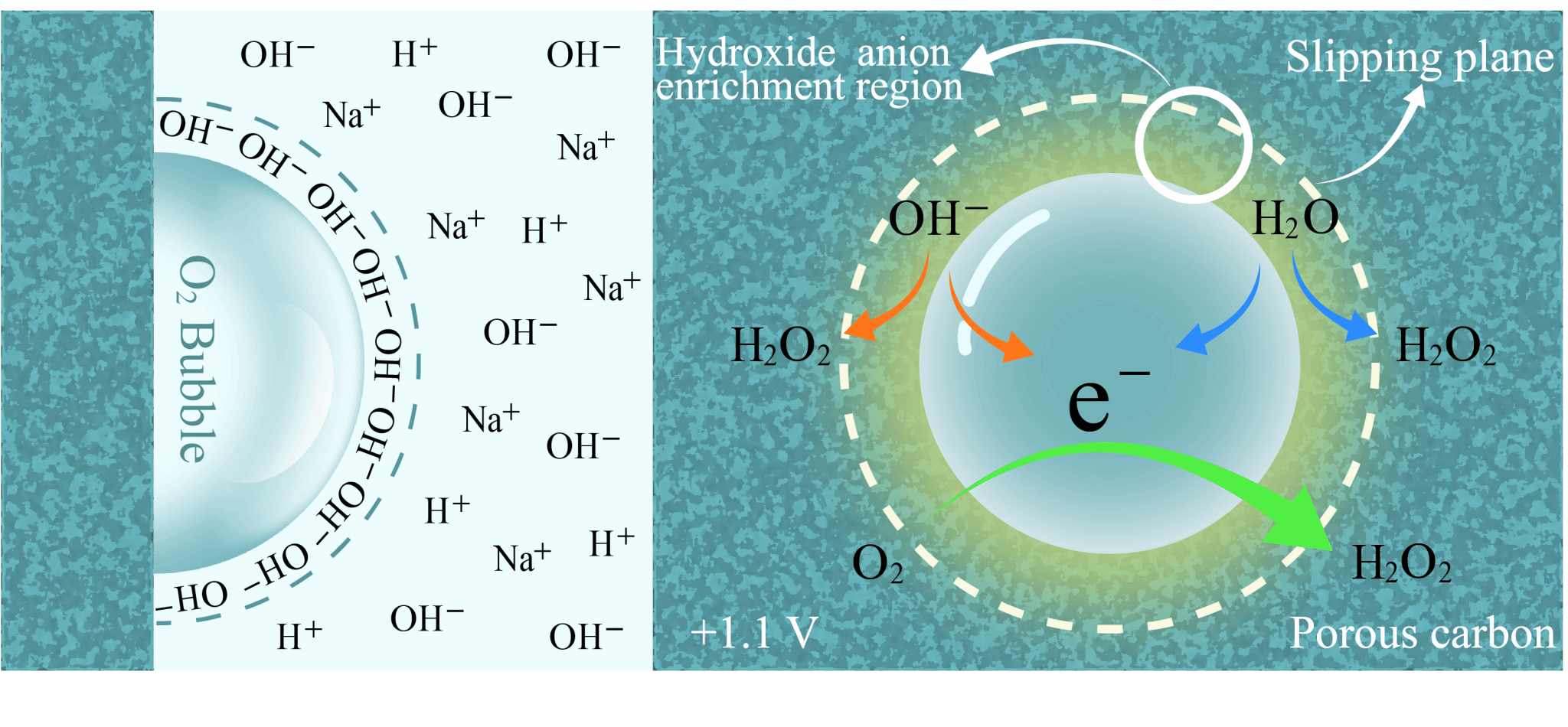
The graphic illustrates the ion distribution and H2O2 generation mechanism at the gas–liquid–solid interface formed by an O2 bubble pinned on a porous carbon electrode. Under an applied potential of +1.1 V, the bubble promotes OH− accumulation, facilitating its oxidation to •OH radicals (red arrow), which subsequently dimerize into H2O2. Meanwhile, water molecules contribute via a two-electron oxidation pathway (blue arrow), aided by the bubble’s hydrophobicity, which suppresses further oxidation. Additionally, surrounding O2 captures electrons released in the process to form H2O2 through a chemical reduction route (green arrow). The synergy of these three pathways enables efficient and catalyst-free electrochemical H2O2 synthesis.
This research was supported by the National Natural Science Foundation of China, Jiangsu Province's “Dengfeng” Talent Program, and the Start-up Fund of Jiangsu University.
Link to the article: https://pubs.acs.org/doi/full/10.1021/jacs.5c05286